Welcome to
MedSafetyWeek
MedSafetyWeek is a global campaign organized by the Iraqi Pharmacovigilance Center in collaboration with the Uppsala Monitoring Centre, along with more than 100 countries worldwide, taking place from November 3 to 9 to promote the reporting of adverse drug reactions in Iraq.


Medicines Safety Awareness
Raising awareness about the importance of reporting adverse drug reactions in Iraq.
A Global Unified Campaign
Participation of more than 100 countries in collaboration with the Uppsala Monitoring Centre.

Engagement of Health Institutions
On-ground activities and social media initiatives to promote the culture of pharmacovigilance.
What is MedSafetyWeek?
MedSafetyWeek is a global campaign organized by the Iraqi Pharmacovigilance Center, in collaboration with the Uppsala Monitoring Centre and more than 100 countries worldwide. It takes place from November 3 to 9, aiming to promote the reporting of adverse drug reactions in Iraq. The campaign includes various activities on social media and in healthcare institutions to raise awareness about the pharmacovigilance system in Iraq.

The Iraqi Pharmacovigilance Center, part of the Technical Affairs Directorate at the Ministry of Health, is responsible for monitoring adverse drug reactions and safety-related issues of medical products in Iraq. The center collects, analyzes, and acts upon these reports to minimize potential risks when necessary.

Alright, how can I contribute?
How can you contribute?
You can support the #MedSafetyWeek campaign by promoting medicine safety awareness and reporting any adverse drug reactions through the pharmacovigilance reporting form.
Follow all campaign updates using the hashtag #MedSafetyWeek.
Spread awareness among your family and friends about medicine safety.
Share and repost content from the official pages.
Report any side effects through the Pharmacovigilance Reporting Form.
Frequently Asked Questions
Enter the basic information: the medicine name, dosage, dates, side effect, and any available test results. Add a contact method for follow-up if needed (kept confidential).
- Suspected side effects from any medicine or vaccine (such as skin rash, injection reactions, dizziness — even if you’re not sure of the cause).
- Quality issues (color or smell changes, damaged packaging, difficulty opening, or reduced effectiveness).
- Medication errors or drug interactions that may affect patient safety.
In case of severe symptoms (such as shortness of breath, chest pain, bleeding, etc.), go to the emergency room immediately.
Anyone (whether a patient, caregiver, or someone close to the patient) can report side effects.
These reports help identify new adverse reactions and gather more information about known ones, improving medicine safety overall.
We may contact you for additional information if needed to help assess the case.
Reports are reviewed by the Pharmacovigilance Center and securely stored in the national database to support medicine safety improvement.
Yes, report any suspected side effect.
Every report makes a difference
Access the form to document side effects and treatment-related issues.
Steps to Install the Form as an App
On Android
1- Visit the form at:
https://vigiflow-eforms.who-umc.org/iq/iqadr
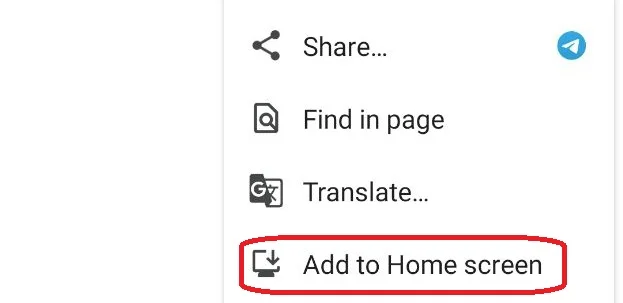
2- Tap the three dots in the corner of your browser
3- Select “Add to Home screen”
4- A new icon will appear on your home screen for quick access.
On iPhone
1- Visit the form at:
https://vigiflow-eforms.who-umc.org/iq/iqadr
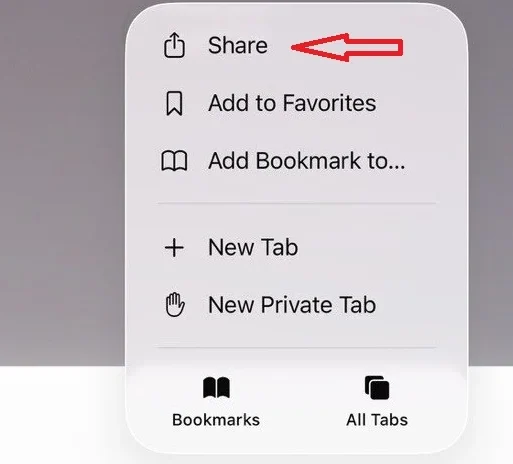
2- Tap the Share button at the bottom of the page
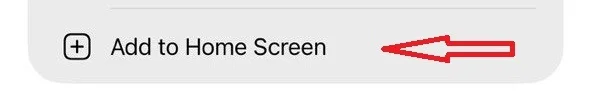
3- Select “Add to Home Screen”
4- A new icon will appear on your home screen for quick access
